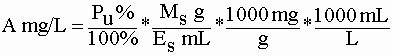Lead in Paint
According to the Centers for Disease Control, lead poisoning is the number one preventable illness in children in the United States. The primary cause is ingestion of lead paint dust and chips. Since 1978, paint offered for sale for household use is supposed to contain less than 0.06% lead in the dried film. About half of houses built before that time are likely to contain lead paint that may be hazardous. In 1992 the state of Connecticut adopted regulations seeking to reduce the hazard to children under the age of six. Health department regulations define an existing painted surface with more than 0.5 percent (wt/wt) lead by laboratory analysis as lead-based paint. When children are exposed to defective lead-based painted surfaces, the regulations require abatement by approved methods.
We will digest paint chips in nitric acid then measure the lead content of the digest using an atomic absorption spectrophotometer (AAS).
Experimental Procedure:
Digestion:
- Weigh to the nearest milligram a sample of paint chips from an area roughly one square inch. Samples should include all layers of paint and be free of adhering substrate. The sample should be between 0.1 and 1.0 g of paint.
- You should also run a digested blank, a duplicate, a digested standard and a spike with each set. (A set would be all the paint samples in a group.)
- To run a digested blank, add 10 mL of acid to an empty flask.
- To run a duplicate, put a second portion of paint in another flask.
- To make a spike, add 2 mL of lead standard (1000 ppm) to a second portion of paint. This does not have to be the same paint as used for the duplicate.
- In a fume hood: Add 10 ml of concentrated nitric acid to each flask. Boil almost to dryness on a hot plate. This will take some time. When the sample is almost dry, the solid matter should be visibly digested. If not, remove flask from the heat. When cool add 10 mL more of concentrated HNO3 and reboil.
- When you have boiled down each portion of acid from step 3, remove the flask from the heat. When the flask is cool enough to touch, add 50 ml of deionized water and two drops concentrated nitric acid. Add boiling chips and allow to boil gently for 30 minutes. Mix thoroughly.
- Transfer to a 50 mL centrifuge tube. Make up to 50 ml with distilled water.
- Centrifuge on high for at least 10 minutes. See AGB for instructions on proper loading of the centrifuge.
Analysis:
- Make up a series of lead standards containing 2, 5, 10, 20, 40 and 80 parts per million (milligrams per liter) lead.
- Run the standards on the atomic absorption spectrophotometer with the detector set to 283 nm. Record the absorbance values (in absorbance units, AU) for each standard. The AU values for the standards can be plotted to establish a standard curve. Calculate a slope and intercept or plug the values into Excel.
- Run the sample extracts. Absorbance values can be read directly from the meter. Any extract which is off scale (ie, above the A value for the highest standard) needs to be diluted and run again. Compare absorbance with the standard curve (use the slope and intercept or the FORECAST function in Excel) to determine milligrams per liter (mg/L) of lead in the sample extracts.
- Calculate percent lead in the paint chips:
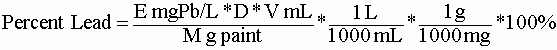
Where:
E = concentration of lead in the extract
D = dilution factor (if any) = (mL final)/(mL orig)
V = volume of extract (usually 50 mL)
M = mass of paint chips
What is a "spike"?
A spike is a sample run in duplicate with a known amount of the analyte of interest added. We hope that the test will show all of the added material (ie, that the sample will not interfere with the test). Suppose that you ran a chloride titration and got a value of 10 mg/L. Then you added chloride so that the chloride concentration was increased in your sample by 100 mg/L. When you ran the test again, you would expect to get a value of 110 mg/L (10 from the sample and 100 from the spike). The amount added is usually about ten times the amount we expect to find naturally in the sample. If we subtract the amount of analyte in the original sample from the total amount found in the spiked sample, we expect to get the amount added to the spike. We divide the result of this subtraction by the amount added and express as a percentage to get the "percent recovery from the spike" or %RS. The %RS is a QC value that can be tracked on a control chart. Ideally, you get 100% but some tests will vary by more than 10% from this.
The %RS is easy to calculate for a water sample:
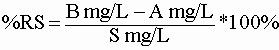
Where A is the amount in the original sample; B is the amount measured in the spiked sample; and S is the amount added as a spike divided by the volume it was added to. In our chloride example, this would be:
(110mg/L-10mg/L)/100mg/L * 100% = 100%
For solid samples such as paint, the calculation is just a bit more complicated as shown below.
Required in Lab Report #5
- USE APPROPRIATE SIGNIFICANT FIGURES IN ALL FINAL RESULTS!
- Title Page; Include
- Title
- Authorís name
- Lab partnersí names
- Date
- Results
- Show your calculations of lead content of paint chips run by you and your partner(s)
- Prepare a letter report to me on your findings; provide information on
- where the samples were from, by whom & when collected
- what the lead concentration of the paint chip sample(s) was
- what the results mean (for example, based on information from the Consumer Products Safety Commission or the EPA)
- you do not need to include the results for the spike or the duplicate in the letter (you will be reporting these on a separate page)
- Calibration Curve
- Prepare an X-Y plot of standard concentration in mgPb/L (x-axis) versus AU (y-axis), including a regression line of the form Pb (mg/L) = A * AU + B (where A is the slope and B is the intercept).
- include the blank and all your standards
- samples are not usually plotted on a standard curve
- Spike Recovery and Relative Percent Difference
- To calculate the percent recovery for the spike, you can use the (B-A)/S formula given above. B is still the concentration in the spiked extract (the mg/L value you get using the standard curve). S is still the amount of added lead divided by the final volume of the spiked extract (for 2 mg added to a 50 mL extract S would be 40 mg/L). A is the tough one. It is calculated using the percent lead (Pu) obtained on the unspiked sample, the mass of paint (Ms) used in the spiked sample, and the volume of the spiked extract (Es):
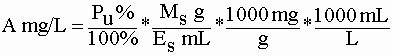
- Calculate the RPD for the duplicate paint sample (as you have done previously).
- Notebook Pages
- you will have a lot of info here; be sure it is neat and complete
Top Home
Anthony Benoit abenoit@trcc.commnet.edu
![]()
![]()